
Rhodium
Chemical reactions
Reaction of rhodium with acidsRhodium does not dissolve in acids, including aqua regia.
Reaction of rhodium with airRhodium does not react with with oxygen, O2, under normal conditions. If heated to 600 °C, rhodium is oxidized to Rh(III) oxide:
4 Rh(s) + 3 O2(g)  2 Rh2O3(s) [dark grey] 2 Rh2O3(s) [dark grey]Reaction of rhodium with halogensRhenium react with fluorine, F2, forming rhodium(VI) fluoride, RhF6. If heated, the hexafluoride is transformed to the tetrameric rhodium(V) fluoride, [RhF5]4.
Rh(s) + 3 F2(g)  RhF6(s) [black] RhF6(s) [black]4 RhF6(s) 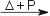 [RhF5]4(s) [dark red] + 2 F2(g) [RhF5]4(s) [dark red] + 2 F2(g)Under dry conditions, rhodium(III) fluoride can be formed: 2 Rh(s) + 3 F2(g)  2 RhF6(s) [red] 2 RhF6(s) [red]Rhenium react with chlorine, Cl2 and bromine, Br2, forming the corresponding rhodium(III) halides: 2 Rh(s) + 3 Cl2(g)  2 RhCl6(s) [red] 2 RhCl6(s) [red]2 Rh(s) + 3 Br2(g)  2 RhBr6(s) [red-brown] 2 RhBr6(s) [red-brown]Reaction of rhodium with waterRhodium does not react with water under normal conditions.
|