
Chlorine
Chemical reactions
Reaction of chlorine with airChlorine, Cl2, does not react with oxygen, O2, and nitrogen, N2.
Chlorine react with carbon monoxide, CO, forming COCl2 [5]. Cl2(g) + CO(g)  COCl2(g) COCl2(g)Reaction of chlorine with basesChlorine, Cl2, reacts with hot aqueous alkali, forming chlorate, ClO3−.
3 Cl2(g) + 6 OH−(aq)  ClO3−(aq) + 5 Cl−(aq) + 3H2O(l) ClO3−(aq) + 5 Cl−(aq) + 3H2O(l)Reaction of chlorine with other halogensFluorine, F2, reacts with chlorine, Cl2, at 225 °C, forming ClF and ClF3.
Cl2(g) + F2(g)  2 ClF(g) 2 ClF(g)Cl2(g) + 3 F2(g)  2 ClF3(g) 2 ClF3(g)Excess fluorine at 350 °C and 225 atmospheres pressure will give ClF5 instead. Cl2(g) + 5 F2(g) 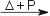 2 ClF5(g) 2 ClF5(g)In gas phase, chlorine, Cl2 will react with bromine, Br2, forming ClBr. Cl2(g) + Br2(g)  2 ClBr(g) 2 ClBr(g)In gas phase, at room temperature, chlorine, Cl2 will react with iodine, I2, forming ClI. Cl2(g) + I2(g)  2 ICl(s) 2 ICl(s)Reaction of chlorine with hydrogenHydrogen reacts with Cl2 forming hydrogen chloride. The reaction is slow at room temperature, and increases in speed with increasing temperatures. The reaction can be explosive under the right conditions [5].
H2(g) + Cl2(g)  2 HCl(g) 2 HCl(g)Reaction of chlorine with metals/metal ionsCadmium reacts directly with Cl2 in aqueous solution [4].
Cd(s) + Cl2(aq)  Cd2+(aq) + 2 Cl−(aq) Cd2+(aq) + 2 Cl−(aq)Calcium reacts with Cl2 forming calcium chloride [5]. Ca(s) + Cl2(g)  CaCl2(s) CaCl2(s)Lithium reacts with Cl2 forming lithium chloride [5]. 2 Li(s) + Cl2(g)  2 LiCl(s) 2 LiCl(s)Magnesium react with Cl2 forming magnesium chloride [5]. Mg(s) + Cl2(g)  MgCl2(s) MgCl2(s)Manganese reacts with Cl2 forming manganese(II)chloride [5]. Mn(s) + Cl2(g)  MnCl2(s) MnCl2(s)Silicon reacts with Cl2 forming silicon chloride [5]. Si(s) + 2 Cl2(g)  SiCl4(l) SiCl4(l)Tin reacts with Cl2 forming tin(IV)chloride [5]. Sn(s) + 2 Cl2(g)  SnCl4(s) SnCl4(s)Reaction of chlorine with phosphorusPhosphorus reacts with excess Cl2 forming phosphorus chloride [5].
P4(s) + 10 Cl2(g)  4 PCl5(s) 4 PCl5(s)with excess phosphorous [5], the reaction is 2 P(s) + 3 Cl2(g)  2 PCl3(s) 2 PCl3(s)PCl3(s) + Cl2(g)  PCl5(s) PCl5(s)Reaction of chlorine with sulfurSulfur reacts with excess chlorine forming sulfur(I)chloride or sulfur(II)chloride [5]:
2 S(s) + Cl2(g)  S2Cl2(s) S2Cl2(s)S(s) + Cl2(g)  SCl2(s) SCl2(s)Chlorine reacts with SO2 forming SO2Cl2 [5]: SO2(g) + F2(g)  SO2F2(g) SO2F2(g)Chlorine reacts with H2S forming HCl [5]: H2S(g) + Cl2(g)  2 HCl(g) + S(s) 2 HCl(g) + S(s)Reaction of chlorine with waterChlorine, Cl2, reacts with water, forming hypochlorite, ClO− [5].
Cl2(g) + H2O(l)  OCl−(aq) + 2 H+(aq) + Cl−(aq) OCl−(aq) + 2 H+(aq) + Cl−(aq) |