
Bromine
Chemical reactions
Reaction of bromine with airBromine, Br2, does not react with oxygen, O2, or nitrogen, N2.
Bromine does react with ozone, O3, at -78 °C, forming bromine(IV) oxide, BrO2. Br2(l) + 2 O3(g)  2 BrO2(s) + O2(g) 2 BrO2(s) + O2(g)Bromine react with carbon monoxide, CO, forming COBr2 [4]. Br2(l) + CO(g)  COBr2(l) COBr2(l)Reaction of bromine with basesBromine, Br2, react with hot aqueous alkali, forming bromate, BrO3−. Only one sixth of the total bromine is converted in this reaction.
3 Br2(g) + 6 OH−(aq)  BrO3−(aq) + 5 Br−(aq) + 3 H2O(l) BrO3−(aq) + 5 Br−(aq) + 3 H2O(l)Reaction of bromine with halogensBromine, Br2, reacts with fluorine, F2, in gas phase, forming BrF. The product is difficult to obtain pure as BrF react with itself, forming Br2, BrF3 and BrF5.
Br2(g) + F2(g)  2 BrF(g) 2 BrF(g)3 BrF(g)  Br2(l) + BrF3(l) Br2(l) + BrF3(l)5 BrF(g)  2 Br2(l) + BrF5(l) 2 Br2(l) + BrF5(l)Using excess fluorine at 150 °C, bromine will react with fluorine forming BrF5. Br2(l) + 5 F2(g)  2 BrF5(l) 2 BrF5(l)Chlorine, Cl2, reacts with bromine, Br2, in gas phase, forming the unstable bromine(I) chloride, ClBr. Cl2(g) + Br2(g)  2 ClBr(g) 2 ClBr(g)Bromine, Br2, reacts with iodine, I2, at room temperature, forming bromine(I) iodide, BrI. Br2(l) + I2(s)  2 IBr(s) 2 IBr(s)Reaction of bromine with hydrogenHydrogen reacts with Br2 forming hydrogen bromide. The reaction is slow at room temperature, and increases in speed with increasing temperatures [4].
H2(g) + Br2(g)  2 HBr(g) 2 HBr(g)Reaction of bromine with metals/metal ionsCd reacts directly with Br2 in aqueous solution and Br2(g) at 450 °C [5].
Cd(s) + Br2(aq)  Cd2+(aq) + 2 Br−(aq) Cd2+(aq) + 2 Br−(aq)Cd(s) + Br2(g) 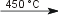 CdBr2(s) [pale yellow] CdBr2(s) [pale yellow]Mn(II)-ions are readily oxidized to MnO2 by brome under alkaline conditions Mn2+(aq) + Br2(aq) + 2 OH−(aq)  MnO2(s) [brown-black] + 2 HBr(aq) MnO2(s) [brown-black] + 2 HBr(aq)Manganese with oxidation steps >2 will be reduced to Mn(II) by Br− under acidic conditions under the formation of Br2, e.g. MnO2(s) + 2 Br−(aq) + 4 H+(aq)  Mn2+(aq) + Br2(aq) + 2 H2O(l) Mn2+(aq) + Br2(aq) + 2 H2O(l)Nickel(II) can be oxidized to nickel(III) using Br2 under alkaline conditions 2 Ni(OH)2(s) + Br2(aq) + 2 OH−(aq)  2 Ni(OH)3(s) + 2 Br−(aq) 2 Ni(OH)3(s) + 2 Br−(aq)Reaction of bromine with phosphorusPhosphorous reacts with excess Br2 forming phosphorous(V)chloride [4].
2 P(s) + 5 Br2(l)  2 PBr5(s) 2 PBr5(s)with excess phosphorous [4], the reaction is 2 P(s) + 3 Br2(l)  2 PBr3(s) 2 PBr3(s)PBr3(s) + Br2(l)  PBr5(s) PBr5(s)Reaction of bromine with sulfurSulfur reacts with excess bromine forming sulfur(I)bromide or sulfur(II)bromide [4]:
2 S(s) + Br2(g)  S2Br2(s) S2Br2(s)S(s) + Br2(g)  SBr2(s) SBr2(s)Bromine reacts with H2S forming HBr [4]: H2S(g) + Br2(l)  2 HBr(g) + S(s) 2 HBr(g) + S(s)Reaction of bromine with waterBromine, Br2, react with water, forming hypobromite, BrO− [4].
Br2(l) + H2O(l)  BrO−(aq) + 2 H+(aq) + Br−(aq) BrO−(aq) + 2 H+(aq) + Br−(aq) |