
Rhenium
Chemical reactions
Reaction of rhenium with acidsRhenium does not dissolve in hydrochloric acid (HCl) and hydrofluoric acid (HF). It does dissolve in nitric acid, HNO3, and concentrated sulphuric acid, H2SO4, forming solutions of perrhenic acid, HReO4.
Reaction of rhenium with airRhenium reacts slowly with oxygen, O2, tarnishing the surface, under normal conditions. If heated, rhenium is oxidized to Re(VII) oxide:
4 Re(s) + 7 O2(g)  2 Re2O7(s) 2 Re2O7(s)Reaction of rhenium with halogensRhenium will react with F2 when heated, forming a mixture of rhenium(VI) fluoride and rhenium(VII) fluoride. At 400 °C and under pressure, only rhenium(VII) fluoride is formed.
Re(s) + 3 F2(g)  ReF6(s) ReF6(s)2 Re(s) + 7 F2(g) 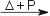 2 ReF7(s) 2 ReF7(s)Reaction of rhenium with waterRhenium reacts with water [1]:
Re(s) + 2 H2O(l)  ReO2(s) + 4 H+(aq) + 4 e− E° = -0.251 V ReO2(s) + 4 H+(aq) + 4 e− E° = -0.251 VRe3+(aq) + 3 H2O(l)  ReO3(s) + 6 H+(aq) + 3 e− E° = -0.318 V ReO3(s) + 6 H+(aq) + 3 e− E° = -0.318 VRe3+(aq) + 4 H2O(l)  ReO42−(aq) + 8 H+(aq) + 3 e− E° = -0.795 V ReO42−(aq) + 8 H+(aq) + 3 e− E° = -0.795 VRe3+(aq) + 4 H2O(l)  ReO4−(aq) + 8 H+(aq) + 4 e− E° = -0.422 V ReO4−(aq) + 8 H+(aq) + 4 e− E° = -0.422 V |