
Osmium
Chemical reactions
Reaction of osmium with airOsmium metal does not normally react with air. When heated, osmium tetraoxide, OsO4 is formed:
Os(s) + 2 O2(g)  OsO2(s) OsO2(s)Reaction of osmium with halogensOsmium will react with F2 under pressure and elevated temperatures. At 600 °C and 400 atmosphere pressure, osmium(VII) fluoride, OsF7, is formed. Under lower temperature and pressure, osmium(VI) fluoride, OsF6, is formed.
2 Os(s) + 7 F2(g) 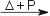 2 OsF7(s) [yellow] 2 OsF7(s) [yellow]Os(s) + 3 F2(g) 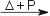 2 OsF6(s) [yellow] 2 OsF6(s) [yellow]For chlorine and bromine, reaction under heat and pressure, gives the corresponding Os(IV) halides: Os(s) + 2 Cl2(g)  OsCl4(s) [red] OsCl4(s) [red]Os(s) + 2 Br2(g)  OsBr4(s) [black] OsBr4(s) [black]Reaction of osmium with waterOsmium reacts with water [1]:
Os(s) + 2 H2O(l)  OsO2(s) + 4 H+(aq) + 4 e− E° = -0.687 V OsO2(s) + 4 H+(aq) + 4 e− E° = -0.687 VOs(s) + 4 H2O(l)  OsO42−(aq) + 8 H+(aq) + 6 e− E° = -0.994 V OsO42−(aq) + 8 H+(aq) + 6 e− E° = -0.994 VOs(s) + 4 H2O(l)  OsO4 + 8 H+(aq) + 8 e− E° = -0.85 V OsO4 + 8 H+(aq) + 8 e− E° = -0.85 V |