
Iodine
Chemical reactions
Reaction of iodine with acidsIodine, I2, reacts with concentrated nitric acid, forming iodic acid [5].
I2(s) + 10 HNO3(aq)  2 HIO3(s) + 10 NO2(g) + 4 H2O(g) 2 HIO3(s) + 10 NO2(g) + 4 H2O(g)Reaction of iodine with airIodine, I2, does not react with oxygen, O2, or nitrogen, N2. It does reat with ozone, O3, forming the unstable yellow I4O9.
Reaction of iodine with basesIodine, I2, reacts with hot aqueous alkali, forming iodate IO3−.
3 I2(s) + 6 OH−(aq)  IO3−(aq) + 5 I−(aq) + 3 H2O(l) IO3−(aq) + 5 I−(aq) + 3 H2O(l)Reaction of iodine with other halogensIodine, I2, reacts with fluorine, F2, at room temperature, forming iodine(V) fluoride. At 250 °C the product is iodine(VII) fluoride. At -45 °C, suspension in CFCl3, iodine(III) fluoride is formed.
I2(s) + 5 F2(g)  2 IF5(l) [colourless] 2 IF5(l) [colourless]I2(g) + 7 F2(g)  2 IF7(g) [colourless] 2 IF7(g) [colourless]I2(s) + 3 F2(g)  2 IF3(s) [yellow] 2 IF3(s) [yellow]Iodine, I2, reacts with bromine, Br2, forming the very unstable, low melting solid iodine(I) bromide. I2(s) + Br2(l)  2 IBr(s) 2 IBr(s)Iodine, I2, reacts with excess chlorine, Cl2, at -80 °C, forming iodine (III) chloride, I2Cl6 (note that the formula is not ICl3 as one would expect). At room temperature, in the presence of water, iodic acid is formed. I2(s) + 3 Cl2(l)  I2Cl6(s) [yellow] I2Cl6(s) [yellow]I2(aq) + 6 H2O(l) + 5 Cl2(g)  2 HIO3(aq) + 10 HCl(g) 2 HIO3(aq) + 10 HCl(g)Reaction of iodine with hydrogenHydrogen reacts with I2 forming hydrogen iodide. The reaction is slow at room temperature, and increases in speed with increasing temperatures [5].
H2(g) + Br2(g)  2 HBr(g) 2 HBr(g)Reaction of iodine with metals/metal ionsSolid Cd does not react with I2(g), but will react with I2(aq). In gas phase Cd and I2 will react forming CdI2. At high temperature and pressure (e.g. a steel bomb) equivalent Cd and I2 will react forming CdI [4].
Cd(s) + I2(aq)  Cd2+(aq) + 2 I−(aq) Cd2+(aq) + 2 I−(aq)Cd(g) + I2(g)  CdI2(g) CdI2(g)2 Cd(g) + I2(g) 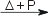 2 CdI(g) 2 CdI(g)Manganese with oxidation steps >2 will be reduced to Mn(II) by I− under acidic conditions under the formation of I2, e.g. MnO2(s) + 2 I−(aq) + 4 H+(aq)  Mn2+(aq) + I2(aq) + 2 H2O(l) Mn2+(aq) + I2(aq) + 2 H2O(l)Reaction of iodine with waterIodine, I2, reacts with water, forming hypoiodite, IO−.
I2(aq) + H2O(l)  IO− + 2 H+(aq) + I−(aq) IO− + 2 H+(aq) + I−(aq) |